Background
In recent years, new therapy strategies such as the antibody-drug conjugate inotuzumab ozogamicin, the bi-specific T-cell engager blinatumomab, and chimeric antigen receptor (CAR) T-cells have been introduced to salvage relapsed precursor B acute lymphoblastic leukemia (ALL). However, recent evidence indicates that these new therapies are ineffective in TP53-aberrant ALL, although the mechanism remains unclear.
TP53 is the most frequently mutated gene in cancer, but in ALL, mutations or deletions affecting TP53 are rare, with an incidence of only 3% at diagnosis. However, at relapse, TP53 aberrations are found in approximately 12% of the patients and predict a very poor outcome. Consequently, relapsed TP53 deleted ALL is now classified as ‘very high risk’. Since p53-mediated apoptosis is an endpoint for many (cytotoxic) drugs, loss of p53 function induces therapy resistance to classical chemotherapeutics. Hence, there is a clear clinical need for more effective strategies to treat TP53 aberrant relapsed ALL.
Methods
Using CRISPR/Cas9, we introduced frameshift mutations in the TP53 gene to generate isogenic wild type and knockout models in TP53 WT ALL cell lines Nalm6 and RCH-ACV. Additionally, we prepared CD19-directed CAR T-cells with a 4-1BB co-stimulatory domain from three healthy donors by isolating, transducing, and expanding CD4+ and CD8+ T-cells separately. With flow cytometry, we assessed survival of p53 WT and p53 KO leukemic cells in the presence of CD4+ and CD8+ CAR T-cells at a 1:1 CD4:CD8 ratio. Moreover, we performed mRNA profiling by qRT-PCR and cell membrane profiling by flow cytometry to determine how loss of p53 affects CAR T mediated killing. Initial findings in cell lines were then validated in patient-derived xenografts.
Results
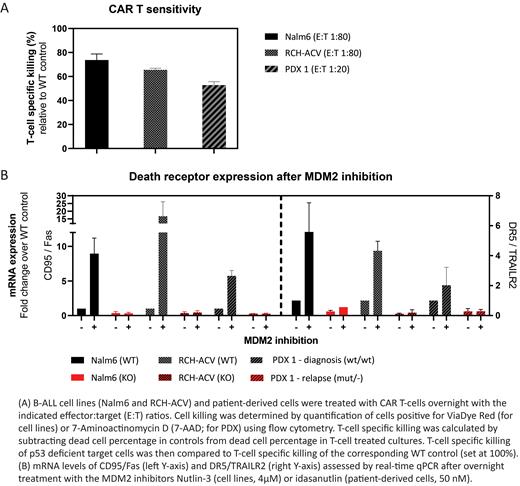
We observed that loss of p53 led to up to a 35% decrease in killing by overnight CAR T treatment in both cell line models (Figure 1A). Although loss of p53 has been linked to reduced cell surface expression of CD19 (Pan et al., 2020, Leukemia), no changes in CD19 expression were observed in response to p53 loss. In search for an explanation for the diminished killing, we performed mRNA profiling and found a reduced expression of death receptors CD95/Fas and DR5/TRAILR2 in p53-deficient cells, which was confirmed at the protein level with flow cytometry (Figure 1B). Indeed, CRISPR/Cas9 mediated loss of CD95/Fas expression in p53 wild type cells reduced CAR T mediated cell killing, consistent with a key role for CD95/Fas in T-cell mediated killing. Conversely, treatment of p53 proficient cells with MDM2 inhibitors induced expression of CD95/Fas and DR5/TRAILR2 (Figure 1B), which translated into enhanced killing of target cells.
Most of these observations have already been recapitulated in a diagnosis-relapse pair of patient-derived cells in which the diagnosis was p53wild typeand the relapse was p53 deficient (Figure 1A-B). We observed lower baseline mRNA expression of CD95/Fas and DR5/TRAILR2 in p53-deficient cells, accompanied by a 50% reduction in killing relative to the p53 wild type controls. Conversely, MDM2 inhibition increased death receptor expression only in p53-proficient cells. The ongoing experiments validating enhanced killing after MDM2 inhibition in patient-derived cells will be presented during the meeting.
Conclusions
Loss of p53 impairs expression of CD95/Fas and DR5/TRAILR2, thereby protecting ALL cells from CAR T induced cell death. In addition, our results indicate that the p53-controlled expression of these receptors may be exploited to increase the efficacy of CAR T-cell therapy in p53-proficient patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal